Blood pH: The Razor Thin Line Keeping You Alive (No Pressure)
If you ever want to feel both wildly impressed by your body and mildly betrayed by it, let’s talk about blood pH.
Your blood has to stay in this tiny, precious range 7.35 to 7.45 or things start going sideways fast. And I don’t mean “oops, I feel a little off.” I mean a shift of about 0.3 can push you toward organ failure. It’s like living your whole life on the world’s narrowest balance beam… while your cells constantly toss acid at you like it’s their hobby.
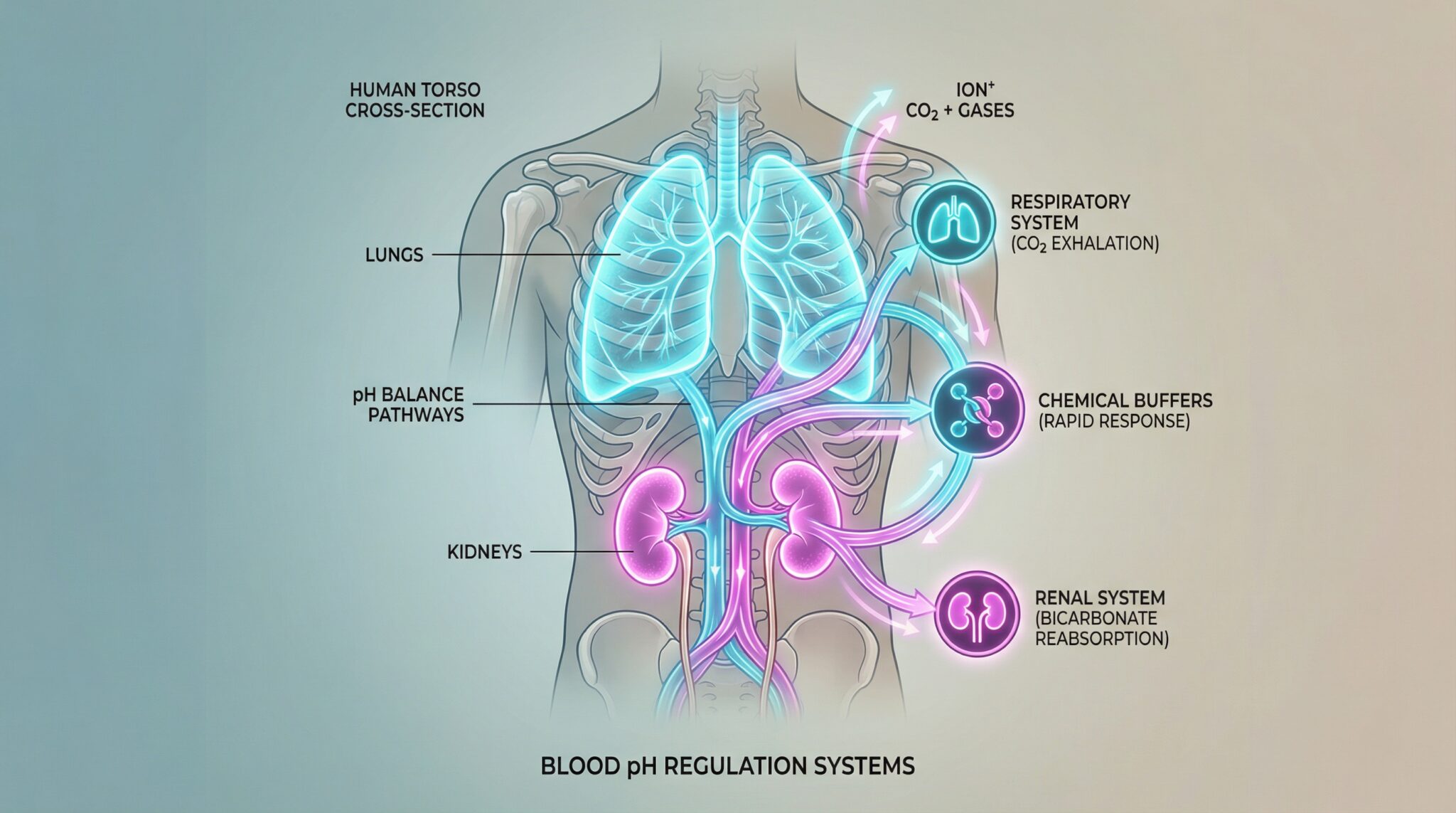
The only reason you’re not thinking about this every day is because your body runs an absolutely relentless, three part “keep me alive” system in the background. Chemical buffers. Lungs. Kidneys. A little relay race of sanity.
So let’s break it down in normal person language with a few “wait, what?!” moments included.
Why your body treats pH like a VIP guest list
Here’s the deal: most of what keeps you alive is driven by proteins enzymes, receptors, all the little biological workers running around doing their jobs. And proteins are… picky. Their shape matters. And pH changes their shape.
So if your pH drifts too far, proteins start acting like someone swapped their coffee for decaf: they just… don’t function right. Nerves, muscles, energy production, clotting everything gets weird.
And meanwhile, your body makes acid all day:
- Burning fuel makes carbon dioxide (CO₂), which is basically “acid potential” in gas form.
- Hard exercise adds lactic acid.
- Fasting or uncontrolled diabetes can make ketoacids.
You’re basically a very busy chemistry lab with excellent janitorial staff.
The pH scale is not “cute little numbers” (it’s dramatic)
pH is logarithmic, which is science speak for “small changes are not small.”
A shift from 7.4 to 7.1 isn’t like turning your thermostat down one degree. It’s more like your “one paint shade darker” becoming “surprise, it’s basically black.” Each step is a tenfold change in acidity.
So your body can’t rely on one flimsy backup plan. It needs layers.
Your three layer pH defense system (aka: buffers, lungs, kidneys)
I like to think of this as a very organized emergency response:
- Chemical buffers act in seconds
- Your lungs adjust in minutes
- Your kidneys take hours to days, but do the long term fixing
And they overlap, which is important. If one system is struggling (hello, lung disease or kidney trouble), the others can try to cover… for a while.
Also, quick vocab so you don’t get sideswiped:
- Acidosis / alkalosis = the process pushing pH down/up
- Acidemia / alkalemia = the actual blood pH is low/high
- ABG = arterial blood gas test (the “let’s look at the actual numbers” test)
Layer 1: Chemical buffers (the instant “hold my purse” reaction)
Buffers are pure chemistry. No brain involvement. No approval process. They just react.
The big star here is bicarbonate (HCO₃⁻), which mops up extra acid. It works closely with CO₂, because your body can convert back and forth between forms and then get rid of CO₂ through your lungs.
Hemoglobin (yes, the oxygen carrying stuff in red blood cells) also helps buffer acid especially after it drops off oxygen in your tissues. Which is honestly convenient, because that’s when your tissues are busiest making acidic byproducts. Your body is nothing if not strategically petty.
There are other backup buffers too (including bone minerals in more chronic situations), but bicarbonate is the main “front line.”
Buffers buy time. They don’t solve the whole problem. For that, you need the exits: lungs and kidneys.
Layer 2: Your lungs (the CO₂ “pressure release valve”)
Every time you breathe out, you’re not just exhaling drama and regrets you’re exhaling CO₂, and that helps control acidity.
- Breathe faster/deeper → you dump more CO₂ → blood becomes less acidic (more alkaline)
- Breathe slower → CO₂ builds up → blood becomes more acidic
This is largely automatic. Your brain and blood vessels have sensors that track CO₂ and pH and adjust your breathing before you even have time to form an opinion about it.
One important nuance: your lungs can compensate pretty quickly, but usually only partway. They can move CO₂ up or down, but if your bicarbonate situation is a mess, your kidneys have to step in.
When breathing itself becomes the problem
- Hyperventilation (anxiety, fever, high altitude, etc.) can cause respiratory alkalosis CO₂ drops too low, blood gets too alkaline. People can feel lightheaded, tingly, crampy. (If you’ve ever had that “hands tingling, mouth numb, am I dying?” panic spiral yeah, that’s part of the physiology.)
- Hypoventilation (opioids, severe COPD, airway blockage) can cause respiratory acidosis CO₂ rises, blood gets more acidic. That can progress from headache/drowsiness to severe impairment.
Layer 3: Kidneys (the slow, steady “deep clean”)
Your kidneys are the long game heroes. They can:
- reclaim bicarbonate so you don’t lose it in urine
- excrete hydrogen ions (acid) in urine
- generate new bicarbonate as needed
This takes time hours to days which is why sudden acid base problems can be so dangerous. Your kidneys can’t sprint. They’re more of a “rebuild the city after the storm” kind of organ.
Also, a very real life complication: electrolytes matter here for how mineral salts influence pH. Low chloride or low potassium can make it harder for your body to correct certain imbalances (like metabolic alkalosis from vomiting or some diuretics). Bodies are annoyingly interconnected like that.
The four acid base disorders (so the doctor speak makes sense)
This is not for self-diagnosing at home this is more like “so you understand what the chart says without Googling at 2 a.m.”
There are four main patterns:
1) Metabolic acidosis
Too much acid or not enough bicarbonate.
- Causes: diabetic ketoacidosis, lactic acidosis, severe diarrhea (bicarb loss), kidney failure
- Classic compensation: deep, fast breathing (Kussmaul respirations) as your lungs try to dump CO₂
2) Metabolic alkalosis
Too much base or not enough acid.
- Causes: prolonged vomiting, some diuretics, certain hormone conditions
- Compensation is limited because your body won’t reduce breathing so much that oxygen drops (your body is many things, but it’s not going to choose “fix alkalosis” over “get oxygen”).
3) Respiratory acidosis
Not breathing out enough CO₂.
- Causes: COPD, pneumonia, sedatives/opioids, airway obstruction
- Acute vs chronic matters: kidneys can compensate better if it’s been going on a while.
4) Respiratory alkalosis
Breathing out too much CO₂.
- Causes: anxiety/panic, fever, high altitude, pulmonary embolism (and others)
ABG results: the “numbers don’t lie” test
If someone’s truly worried about acid base status, they don’t use vibes. They use an ABG (arterial blood gas).
An ABG looks at:
- pH
- PaCO₂ (respiratory side)
- HCO₃⁻ (metabolic side)
- oxygen levels too
A very simplified way clinicians interpret it is:
- Is the pH low or high? (acidemia vs alkalemia)
- Is CO₂ driving it (respiratory) or is bicarbonate driving it (metabolic)?
- Is the other system compensating the way you’d expect?
And yes, it gets more complex in real life, especially when multiple issues are happening at once.
Why urine pH strips don’t tell you what you think they tell you
Urine pH is basically “what your kidneys are dumping today,” which can change with hydration and diet. It does not reliably tell you what your blood pH is doing.
So no, peeing on a strip and declaring yourself “alkaline” doesn’t mean your internal chemistry is thriving. It means your kidneys are doing kidney things.
Red flags: when this is not a “wait and see” situation
If you or someone else has any of the following, treat it as urgent:
- confusion or altered consciousness
- seizures or passing out
- severe shortness of breath
- blue lips or fingernails
- sustained muscle spasms/locking up
- irregular heartbeat with other symptoms
Also: blood pH below ~6.8 or above ~7.8 cannot support life. This is not a “try lemon water” moment.
“So what can I actually do?” (aka: the practical part)
If you’re generally healthy, your body handles pH beautifully without you micromanaging it. But you can support the systems that do the work:
- Hydrate: kidneys need fluid to filter and excrete waste effectively. (You don’t have to drown yourself. Just don’t live in a state of constant dehydration.)
- Don’t neglect minerals: potassium, magnesium, calcium these aren’t “pH boosters,” but they help your body run the machinery that keeps things stable.
- Treat the real problems: uncontrolled diabetes, kidney disease, chronic lung issues those are the things that actually disrupt pH. Not whether you ate tomatoes.
About the “alkaline diet”…
You cannot meaningfully shift your blood pH with food because diet cannot shift blood pH in any healthy, controlled way. Your body will fight you like a toddler refusing socks.
Can diet affect urine pH? Sure. Can it fix diabetic ketoacidosis or kidney failure? Absolutely not.
Blood pH is not a wellness trend. It’s a tightly regulated survival setting.
If you take nothing else from this: your body is doing constant, invisible work to keep you in that 7.35-7.45 sweet spot. So yes drink water, eat like a reasonable person, manage chronic conditions, and maybe take a second to appreciate the fact that your lungs and kidneys are basically running a 24/7 emergency response team with zero applause.